"Spot-On" Flea & Tick Products Alleged to Cause Serious Harm to Dogs and Cats
Pet Pharmaceutical Industry Under Fire in Multiple New Jersey Class Actions
OVER 75,000 COMPLAINTS REPORTED TO EPA ASPCA CONNECTION
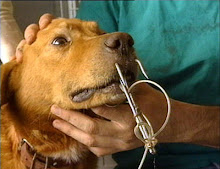
Yet another class action was filed this week in federal court in Newark, New Jersey, in the District of New Jersey, against a manufacturer of "Spot-On" Flea and Tick products, neurotoxin pesticides placed directly on the skin of millions of household dogs and cats across the United States.
There is a growing sense of moral outrage and alarming statistics from consumers and pet advocates alike after over 75,000 complaints about the products have been reported to the EPA and nothing has been done to change the advertising, marketing or labeling of the products to alert pet owners of the possible serious side-effects. What's chilling is that those numbers only reflect what has been reported, the actual number of incidents may be much, much higher.
"Spot-On" products generally do not say in their advertising or marketing or even EPA mandated labeling that the products may cause death or other serious side-effects, including paralysis, seizures and ulcerated skin lesions. Consumers are largely unaware of these possibilities when applying the products to their dogs and cats.
Michael Green of Green & Associates, LLC, one of the attorneys that filed the New Jersey class actions, stated, "I've received a number of phone calls from pet owners in the middle of the night, where they are traumatized after their dog or cat has just died in their arms after they placed these pesticides on them. These are innocent pets who were trusting their owner to do the right thing for them. The owners are horrified to think that they gave their pet something that killed them, often in an excruciatingly painful way. Most tragic are those stories where the owner had one pet die only to put it on another pet after being assured there was no connection to the product and have that pet die also. I've gotten a few of those calls too. Unfortunately, the law treats our dogs and cats as just chattel, but if you ask any of these pet owners whose pet died, their dog or cat was a member of their family."
Sold By Major Retailers and Veterinarians
The "Spot-On" tick and flea business is estimated to be an $8 billion a year industry with the products primarily sold by veterinarians and pet store retailers such as PetCo and PetSmart.
This week, PetArmor, a new "Spot-On" flea and tick product for dogs and cats, is being served with the complaint filed against it. PetArmor was launched on April 20, 2011 in a press conference with the ASPCA. The product has been featured in television ads and is sold in major retailer WalMart. It is described by the manufacturer, FidoPharm, as containing the same active ingredients as Frontline, a well-known veterinarian recommended product.
Other "Spot-On" products sued in the class actions in New Jersey, beginning in January 2010, include Frontline, manufactured by Merial Limited, and those manufactured and sold by the other major animal pharmaceutical corporations Sergeant's Pet Care Products, Inc., Hartz Mountain Corporation, Bayer Healthcare, LLC, Farnum Companies, Wellmark International, Inc. and Summit VetPharm, LLC.
"Spot-On" Flea and Tick products are now recommended for virtually all dogs and cats, no matter the locale or specific risk. The Spot-On industry and vets who profit in the billions of dollars have not been called to account as to whether the risk vs. benefits really necessitate that common household pets across this country, and the children and other humans in these households, need to be exposed to these pesticides and neurotoxins 24/7. Studies have shown that the pesticides are detectible on the hands after petting.
EPA Complaints
Over 75,000 complaints to the EPA have been made since 2008 regarding these products including complaints of death and other serious side-effects even without a centralized poison reporting center, something the Humane Society is now calling for. In April of 2009, the Environmental Protection Agency (EPA) issued an advisory and reported that there was a noticeable increase in the number of adverse pet reactions involving the "Spot-On" pesticides which act as neurotoxins.
Nexus Between ASPCA and "Spot-On" Industry
FidoPharm enlisted the aid of the ASPCA in launching an awareness of their "Spot-On" product PetArmor. However, the class action complaint alleges a nexus of relationships between executives of the "Spot-On" product manufacturers and the ASPCA, heretofore known to the public as a non-profit entity established to prevent cruelty to animals.
The ASPCA has selected FidoPharm as its official tick and flea sponsor in its adoption centers and clinics. FidoPharm in turn has donated product and $100,000 to the ASPCA. John Preston, the current chairman of FidoPharm's parent, was the founding executive of Merial, the maker of Frontline.
Dr. Steven R. Hanson, the Senior Vice President and head of the ASPCA's Poison Control Center, where adverse reports of "Spot-On" products may be made, is a former director of Wellmark. Hartz is a corporate donor to the ASPCA and named Dr. Hanson "Veterinarian of the Year." The complaint alleges that calls into the Poison Control Center regarding Hartz "Spot-On" products are redirected to Hartz, allowing Hartz to directly manage any of these adverse events.
In addition, the ASPCA has a for-profit arm, APCC Consulting Services, that provides professional services to animal pharmaceutical corporations, including consultation on product liability. Hartz has been a client of the APCC.
Finally, the ASPCA in a press release, in response to the 2009 EPA advisory regarding "Spot-On" products, quoted Dr. Hanson as stating the products should continue to be used.
The plaintiffs of the class actions allege that the pesticides in the "Spot-On" products cause death, paralysis, seizures and skin lesions to their pets and that these adverse affects are not stated in the advertising or marketing to pet owners.
More information regarding the class action lawsuits filed against FidoPharm and others may be obtained at www.spotoncomplaints.com
Contact: Michael S. Green, Esq., Green & Associates, LLC
Tel: 732-390-0480, Email: green@msgreenlaw.com
SOURCE Green & Associates, LLC
Thursday, December 8, 2011
Subscribe to:
Post Comments (Atom)
















2 comments:
Thank you for an insightful and useful post. The web is a better place with you writing!
Pet sitting Frisco
Doing some research for a post of my own on this - update -- and did you know that the US EPA noted that the VAST MAJORITY of these deaths and illnesses results from people actually applying the product incorrectly?
I am NOT a spokesperson for any organization... just a blogger like you doing the job!!
JL Smith
Post a Comment